We all have genes that don't work properly. In most cases the other copy of the gene makes up for the one that does not work properly and we are healthy. A problem only arises if we meet someone else who has a non-working copy of the same gene and we have a child who inherits two non-working copies of that gene. This is called recessive inheritance.
Sometimes if one of our genes is not working properly the other copy of the gene cannot make up for it and that causes a condition or an increased risk of developing a condition. Each time we have a child we randomly pass on one copy of each gene. If the child inherits the copy that doesn't work properly, they too may develop the condition. This is called dominant inheritance.
In 1997, we studied a large family that came from a small town in Southern Italy in which PD was inherited from parent to child (dominant inheritance). Researchers found the gene that caused their inherited Parkinson's Disease and it coded for a protein called alpha-synuclein.
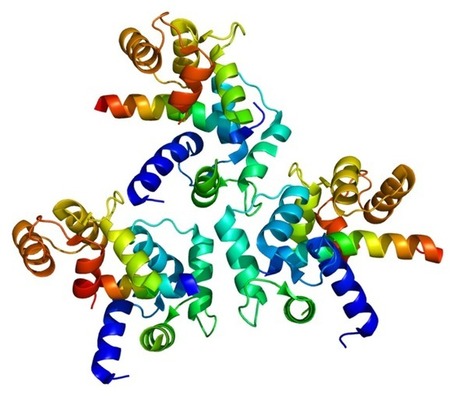 A research team from the Taub Institute at CUMC (Columbia University Medical Center) managed to identify the main mechanism that causes the progressive movement disorder in sporadic Parkinson’s disease patients.
heritable forms of Parkinson’s disease have shown that a very important role in the onset and development of the disease is played by a protein called alpha-synuclein. Patients that have a higher number of alpha-synuclein gene copies will produce a higher quantity of this protein, thus damaging their neurons.
Parkinson’s patients also show an excess of alpha-synuclein aggregates in the brain.
A research team from the Taub Institute at CUMC (Columbia University Medical Center) managed to identify the main mechanism that causes the progressive movement disorder in sporadic Parkinson’s disease patients.
heritable forms of Parkinson’s disease have shown that a very important role in the onset and development of the disease is played by a protein called alpha-synuclein. Patients that have a higher number of alpha-synuclein gene copies will produce a higher quantity of this protein, thus damaging their neurons.
Parkinson’s patients also show an excess of alpha-synuclein aggregates in the brain.If one studies the brains of people with PD after they die, one can see tiny little accumulations of protein called Lewy Bodies (named after the doctor who first found them). Research has shown that there is a large amount of alpha-synuclein protein in the Lewy Bodies of people who have non-inherited PD as well as in the brains of people who have inherited PD. This immediately told us that alpha-synuclein played an important role in all forms of PD and we are still doing a lot of research to better understand this role.
. The longer forms of the alpha-synuclein gene were found to be specific to human patients, as well as some of the genetic variants that are in close connection to the onset of the disease. The research team also showed that if patients are exposed to environmental toxins that are related to Parkinson’s disease, there is an increase in alpha-synuclein. Currently, seven genes that cause some form of Parkinson's disease have been identified. Mutations (changes) in three known genes called SNCA (PARK1), UCHL1 (PARK 5), and LRRK2 (PARK8) and another mapped gene (PARK3) have been reported in families with dominant inheritance. Mutations in three known genes, PARK2 (PARK2), PARK7 (PARK7), and PINK1 (PARK6) have been found in affected individuals who had siblings with the condition but whose parents did not have Parkinson's disease (recessive inheritance). There is some research to suggest that these genes are also involved in early-onset Parkinson's disease (diagnosed before the age of 30) or in dominantly inherited Parkinson's disease but it is too early yet to be certain.
In most cases inheriting a non-working copy of a single gene will not cause someone to develop Parkinson's disease. many other complicating factors such as additional genes and environmental factors determine who will get the condition, when they get it and how it affects them.
By reprogramming skin cells from Parkinson's disease patients with a known genetic mutation, researchers at the Salk Institute for Biological Studies have identified damage to neural stem cells as a powerful player in the disease. The findings, reported online October 17, 2012 in Nature, may lead to new ways to diagnose and treat the disease. The scientists found that a common mutation to a gene that produce the enzyme LRRK2, which is responsible for both familial and sporadic cases of Parkinson's disease, deforms the membrane surrounding the nucleus of a neural stem cell. Damaging the nuclear architecture leads to destruction of these powerful cells, as well as their decreased ability to spawn functional neurons, such as the ones that respond to dopamine.
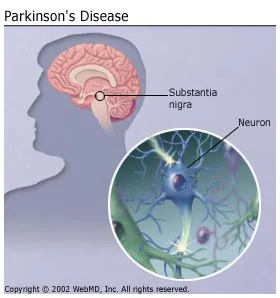
This discovery helps explain how Parkinson's disease, which has been traditionally associated with loss of neurons that produce dopamine and subsequent motor impairment, could lead to locomotor dysfunction and other common non-motor manifestations,such as depression and anxiety.
Its possible to use targeted gene-editing technologies to correct the mutation in patient's nuclear stem cells. This genetic correction repaired the disorganization of the nuclear envelope, and improved overall survival and functioning of the neural stem cells. To be able to chemically inhibit damage to the nucleus, producing the same results seen with genetic correction. This opens the door for drug treatment of Parkinson's disease patients who have this genetic mutation, The new finding may also help clinicians better diagnose this form of Parkinson's disease. Due to the striking appearance in patient samples, nuclear deformation parameters could add to the pool of diagnostic features for Parkinson's disease. The new finding may also help clinicians better diagnose this form of Parkinson's disease. Its the first time to our knowledge that human neural stem cells have been shown to be affected during Parkinson's pathology due to aberrant LRRK2.
The study shows that these reprogramming technologies are very useful for modeling disease as well as dysfunction caused by aging.
Alternatively new compounds target and shut a relatively rare membrane protein that allows the destructive calcium to flood into dopamine neurons. Previously published research showed that calcium entry through this protein stresses dopamine neurons, potentially leading to premature aging and death. The earlier research identified the precise protein involved—the Cav1.3 channel.
"These are the first compounds to selectively target this channel,” says D. James Surmeier, chair of physiology at Northwestern University Feinberg School of Medicine. “By shutting down the channel, we should be able to slow the progression of the disease or significantly reduce the risk that anyone would get Parkinson’s disease if they take this drugearly enough.”
 As reported in the October 23 Nature Communications, the compounds work in a similar way to the drug isradipine, for which a Phase 2 national clinical trial with Parkinson’s patients—led by Northwestern Medicine neurologist Tanya Simuni—was recently completed. But because isradipine interacts with other channels found in the walls of blood vessels, it can’t be used in a high enough concentration to be highly effective for Parkinson’s disease.
As reported in the October 23 Nature Communications, the compounds work in a similar way to the drug isradipine, for which a Phase 2 national clinical trial with Parkinson’s patients—led by Northwestern Medicine neurologist Tanya Simuni—was recently completed. But because isradipine interacts with other channels found in the walls of blood vessels, it can’t be used in a high enough concentration to be highly effective for Parkinson’s disease.
For the next step, the Northwestern team has to improve the pharmacology of the compounds to make them suitable for human use, test them on animals, and move to a Phase 1 clinical trial.
 The final technique if drugs don't work, is extradural motor cortex stimulation (EMCS), may provide a less-invasive alternative to electrical deep brain stimulation (DBS) for some patients with the movement disorder Parkinson's disease. The study was led by Dr. Beatrice Cioni of Catholic University, Rome.
The researchers evaluated EMCS in nine patients with Parkinson's disease. Over the past decade, DBS using electrodes implanted in specific areas within the brain has become an accepted treatment for Parkinson's disease. In the EMCS technique, a relatively simple surgical procedure is performed to place a strip of four electrodes in an "extradural" location—on top of the tough membrane (dura) lining the brain. The electrodes were placed over a brain area called the motor cortex, which governs voluntary muscle movements. The study was designed to demonstrate the safety of the EMCS approach, and to provide preliminary information on its effectiveness in relieving the various types of movement abnormalities in Parkinson's disease. The electrode placement procedure and subsequent electrical stimulation were safe, with no surgical complications or other adverse events.
The final technique if drugs don't work, is extradural motor cortex stimulation (EMCS), may provide a less-invasive alternative to electrical deep brain stimulation (DBS) for some patients with the movement disorder Parkinson's disease. The study was led by Dr. Beatrice Cioni of Catholic University, Rome.
The researchers evaluated EMCS in nine patients with Parkinson's disease. Over the past decade, DBS using electrodes implanted in specific areas within the brain has become an accepted treatment for Parkinson's disease. In the EMCS technique, a relatively simple surgical procedure is performed to place a strip of four electrodes in an "extradural" location—on top of the tough membrane (dura) lining the brain. The electrodes were placed over a brain area called the motor cortex, which governs voluntary muscle movements. The study was designed to demonstrate the safety of the EMCS approach, and to provide preliminary information on its effectiveness in relieving the various types of movement abnormalities in Parkinson's disease. The electrode placement procedure and subsequent electrical stimulation were safe, with no surgical complications or other adverse events.In particular, the patients had no changes in intellectual function or behavior and no seizures or other signs of epilepsy. Extradural stimulation led to small but significant and lasting improvements in control of voluntary movement. After one year, motor symptoms improved by an average of 13 percent on a standard Parkinson's disease rating scale, while the patient was off medications. Although DBS is an effective treatment for Parkinson's disease, it's not appropriate for all patients. Some patients have health conditions or old age that would make surgery for electrode placement too risky. Other patients—including four of the nine patients in the new study—are eligible for DBS but don't want to undergo electrode placement surgery.
According to certain web sites, family history of PD in a first degree relative is seen in about 15% of people with PD. There is a 2x risk of developing PD if you have a family history of PD in a first degree relative. Although increased, this risk is still relatively low. The study also found elevated levels of the alpha-synuclein elongated transcripts in the blood of a group of patients with sporadic Parkinson's, compared with unaffected controls. This would suggest that a test for alpha-synuclein may serve as a biomarker for the disease.
Possible early detection for Parkinson's Disease, would lead to preventative actions. The possible gene reprograming offers a non invasive opportunity of a possible cure. Although another technique is calcium entry of a protein might Cav1.3 channel. Shutting it down might prove a slow progression or a cure. Its is still too early for research to come up with a cure and that avenues of gene manipulation or compound cures can resolve the almost hidden causes to Parkinson's. The last resort would be a surgical approach as deep brail stimulation offers a comfort to those who would risk the dangers for the sake of mobility. Having pace maker for the brain in my view seems a bit cumbersome as the technology hasn't shrunk the equipment. Instead the current research in a non invasive cure, continues to offer hope. Perhaps in the future it may be a matter of popping a pill to prevent a neurodegenerative disease...




My husband was diagnosed with MND ALS (amyotrophic lateral sclerosis) when he was 69 years old 6 years ago. The Rilutek (riluzole) did very little to help him. The medical team did even less. His decline was rapid and devastating. The psychological support from the medical center was non-existent and if it were not for totalcureherbsfoundation. com and the sensitive cure of their herbal formula he would have been not been alive today,there was significant improvement in the first 4 weeks of usage that gave us hope that he will be alive,His doctor put him on riluzole, letting us know there was no cure until we gave try on total cure herbal supplement that cure him totally form this disease after 15 weeks of his usage.there is nothing positive about cure ALS condition except for their herbal treatment .
ReplyDeleteGreat blog you haave
ReplyDelete