 But if a recent report in the Economist is right, there may be a viable alternative — a rather unassuming one that is extremely cheap and in relative abundance: liquid nitrogen. Because liquid nitrogen is dense and capable of storing considerable amounts of energy per unit volume, cars should be able to run a fair distance on the stuff. And weight for weight, it also packs as much energy as lithium-ion batteries used in laptops, mobile phones, and electric cars. And in fact, its performance is predicted to be comparable to that of an electric car's.
But if a recent report in the Economist is right, there may be a viable alternative — a rather unassuming one that is extremely cheap and in relative abundance: liquid nitrogen. Because liquid nitrogen is dense and capable of storing considerable amounts of energy per unit volume, cars should be able to run a fair distance on the stuff. And weight for weight, it also packs as much energy as lithium-ion batteries used in laptops, mobile phones, and electric cars. And in fact, its performance is predicted to be comparable to that of an electric car's.The big difference is that a liquid-nitrogen car is likely to be considerably cheaper to build than an electric vehicle. For one thing, its engine does not have to cope with high temperatures—and could therefore be fabricated out of cheap alloys or even plastics. For another, because it needs no bulky traction batteries, it would be lighter and cheaper still than an electric vehicle. At present, lithium-ion battery packs for electric vehicles cost between $500 and $600 a kilowatt-hour.
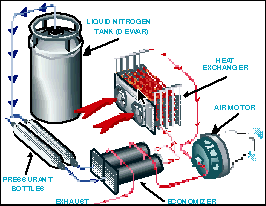
With a $360,000 U.S. Department of Energy grant, UW researchers and students have built a LN2000 prototype from a converted Grumman Kubvan mail truck. Aside from the Husky decals, the vehicle looks much like any other postal truck on the outside. But open up the back and one finds insulated tanks, piping, hoses and gauges more befitting a research laboratory than an automobile. Under the hood is a 15 horsepower air motor originally designed to power a winch for raising ship anchors. And instead of generating plumes of foul exhaust, the LN2000 emits cold nitrogen gas which freezes water vapor in the air to form small clouds behind the vehicle.
The LN2000's heat exchanger pulls liquid nitrogen from an insulated fuel tank through a series of aluminum tubing coils and specially designed pipes. Engine exhaust and outside air are circulated around the coils and pipes to gradually warm up the nitrogen from a minus 320 F liquid to an ambient-temperature gas. The heat exchanger is like the radiator of a car, but it acts in the opposite way. Instead of using air to cool water, it uses air to boil liquid nitrogen into nitrogen gas. The conversion from liquid to gas expands the volume of the nitrogen 700 times, building sufficient pressure to turn an air motor much like pressure from burning gasoline drives an internal combustion engine.
 While the reliance of internal combustion engines on non-renewable fuels has prompted automotive engineers to make car motors more efficient over the years, air motors have had the luxury of remaining fabulously inefficient. As a result, the motor used in the LN2000 prototype gives gas guzzling new meaning by consuming about five gallons of nitrogen fuel per mile. Plus it musters a top speed of only 22 m.p.h. and chugs laboriously up hills.
While the reliance of internal combustion engines on non-renewable fuels has prompted automotive engineers to make car motors more efficient over the years, air motors have had the luxury of remaining fabulously inefficient. As a result, the motor used in the LN2000 prototype gives gas guzzling new meaning by consuming about five gallons of nitrogen fuel per mile. Plus it musters a top speed of only 22 m.p.h. and chugs laboriously up hills.Liquid nitrogen is a by-product of the industrial process for making liquid oxygen. Because there is four times as much nitrogen as oxygen in air, there is inevitably a glut of the stuff—so much so, liquid nitrogen sells in America for a tenth of the price of milk.
 An invention made by an independent British engineer called Peter Dearman dispenses with the costly heat exchanger that is needed to vaporize the liquid nitrogen quickly. Instead, a small amount of water and anti-freeze (eg, methanol) is injected into the cylinder just as the liquid nitrogen is drawn in, causing it to boil and expand rapidly—thereby forcing the piston down inside the the cylinder. “Without that,” says Mr Dearman, “you had to have a multi-stage engine, which is cumbersome, inefficient and expensive.”
An invention made by an independent British engineer called Peter Dearman dispenses with the costly heat exchanger that is needed to vaporize the liquid nitrogen quickly. Instead, a small amount of water and anti-freeze (eg, methanol) is injected into the cylinder just as the liquid nitrogen is drawn in, causing it to boil and expand rapidly—thereby forcing the piston down inside the the cylinder. “Without that,” says Mr Dearman, “you had to have a multi-stage engine, which is cumbersome, inefficient and expensive.”At -196C, air becomes a liquid. One litre of liquid air is “manufactured” by compressing and cooling 710 litres of air. Liquefying air uses energy, i.e. ‘stores’ it. Storing the liquid air is straightforward as it can be held in a non-pressurised insulated tank; it is not flammable (it is in fact classed as a lower hazard than diesel, hydrogen or battery chemicals); it can be produced and stored on site or distributed by pipeline or road tanker.
To turn the liquid air back to gas, simply apply “environmental heat” (anything warmer than -196C). If you do this in a confined space, i.e. inside the cylinder of an engine, the result is high pressure air (now in a normal gaseous state) which can be used to power a vehicle (or a static generator). The engine exhausts cold air back to the environment.
storing renewable energy is being tested in the UK by a cryogenic company, Highview Power Storage, whose liquid air energy storage could be up to 70% efficient and cost just $1,000 per kilowatt.
 The Highview unit would use an unused excess at times of low demand to power refrigeration units to cool air to around -190 °C – which turns air into liquid nitrogen for cryogenic energy storage.
Once the refrigerators, powered by the renewable energy, have chilled the air to a liquid, it is then stored in a tank at ambient pressure – about 1 bar. When electricity is needed, the liquid air is subjected to a pressure of 70 bars and warmed in a heat exchanger.
Just allowing the liquid air to warm again produces a high-pressure gas that can drive a turbine to generate electricity. Using just ambient air heat to warm it, the process recovers around 50% of the electricity that is fed in, but it becomes up to 70% efficient if a source of heat is available. Waste heat could be harnessed inexpensively from a nearby industrial or power plant, and used to heat the cooled air to warmer-than-air temperature for a power boost.
The Highview unit would use an unused excess at times of low demand to power refrigeration units to cool air to around -190 °C – which turns air into liquid nitrogen for cryogenic energy storage.
Once the refrigerators, powered by the renewable energy, have chilled the air to a liquid, it is then stored in a tank at ambient pressure – about 1 bar. When electricity is needed, the liquid air is subjected to a pressure of 70 bars and warmed in a heat exchanger.
Just allowing the liquid air to warm again produces a high-pressure gas that can drive a turbine to generate electricity. Using just ambient air heat to warm it, the process recovers around 50% of the electricity that is fed in, but it becomes up to 70% efficient if a source of heat is available. Waste heat could be harnessed inexpensively from a nearby industrial or power plant, and used to heat the cooled air to warmer-than-air temperature for a power boost.
A 300 kilowatt cryogenic energy storage system has been tested for the last nine months, storing and supplying electricity to the UK grid. It has proved proof of concept and that the process is economical, as the supplies needed are inexpensive. Next, Highview plans to build a 3.5 MW, commercial-scale system by late 2012, building it up into an 8 to 10 megawatt storage plant by early 2014. Till now, the company has been buying the supplies, but it has now added an on-site liquefaction plant, and will begin producing its own liquid air or cryogenic liquid nitrogen from late March, which will reduce costs.
The great potential for liquid nitrogen could offer large scale storage for power, not only as a alternative fuel to power a car but its properties offer a use as a coolant for the cryo industry. This reusable fuel is safe and environmentally friendly idea might offer a good option to fossil fuel energy. In the slow transition of unclean fuels to clean energy it might be a good time to rethink the supply and demand cycle of electrical power....




No comments:
Post a Comment